Peri-implant disease diagnosis is as fundamental as controversial. Although the progress made during the last decades, it’s still hard to find univocal definitions and unambiguous diagnostic criteria.8,14,30
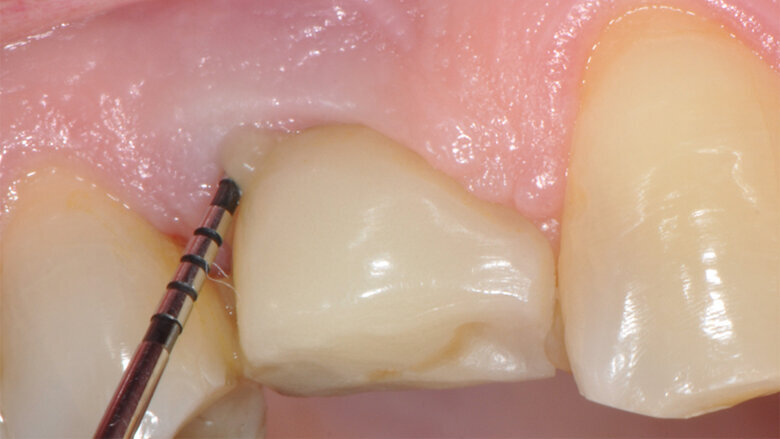
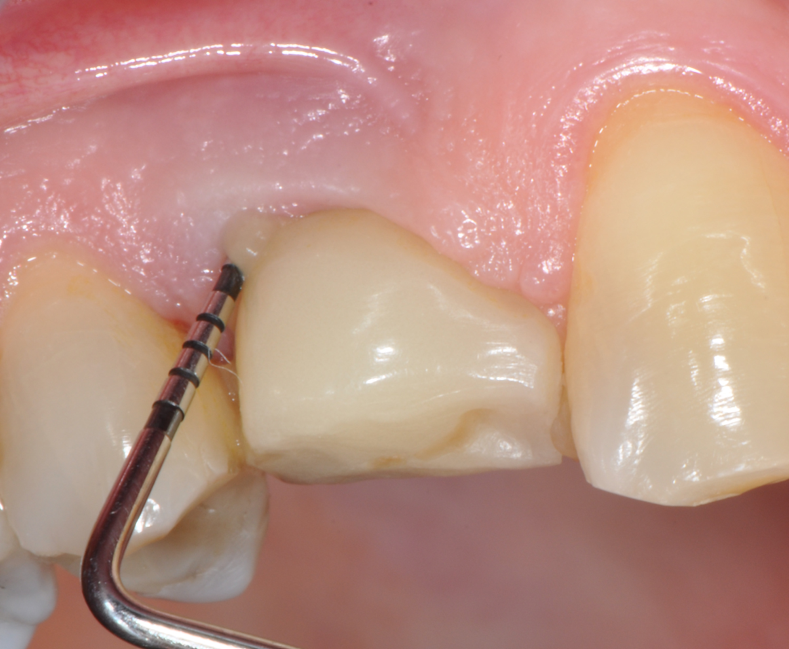
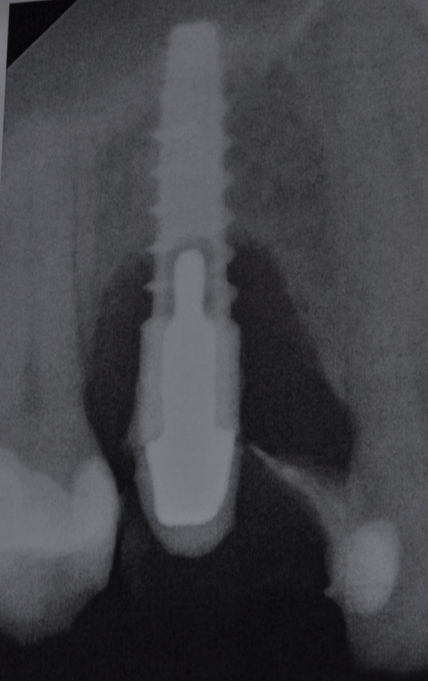
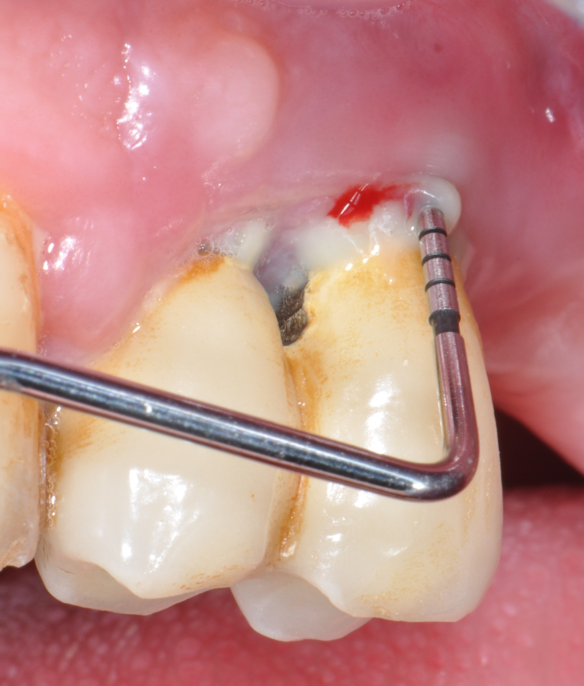
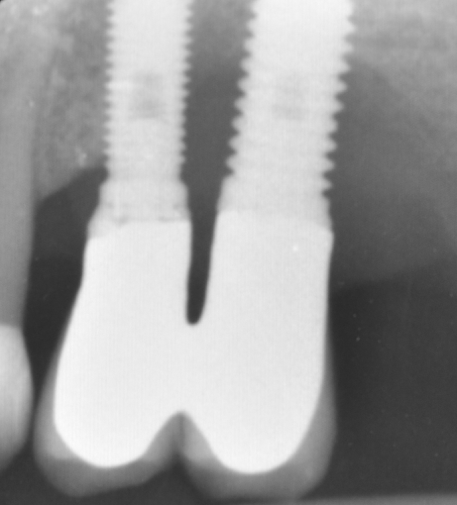
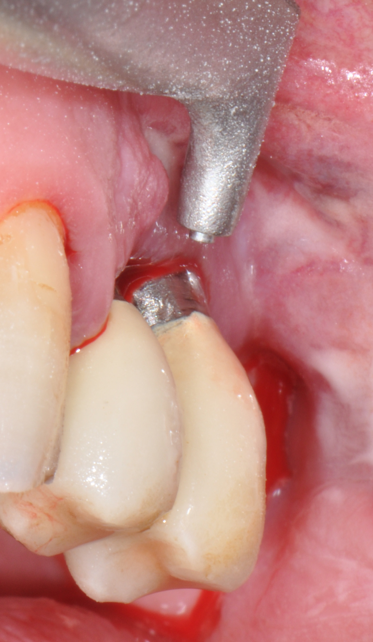
The parameters used to define peri-implant disease usually are: Probing Depth (PD), Crestal Bone Loss (CBL), Bleeding on Probing (BOP) and presence of suppuration and/or fistula.9 Peri-implant mucositis is characterised by soft tissues inflammation witnessed by BOP with or without PD deepening but no effects on the crestal bone while peri-implantitis is characterised by CBL, BOP alone or in conjunction with pus, with or without PD deepening. (Figs. 1, 2 and 3) display the diagnostic steps of a case of peri-implantitis. While mucositis allows a complete healing, peri-implantitis is not reversible.12
PD sets the first controversial point in diagnosis:the sulcus around implants can be consideredsurgically created since it will correspond to the deepness of implant positioning,the quantity of soft tissues and to the length of theabutments. Given that, we cannot easily put a line between “health” and “disease” PD as we do for natural elements.26 It’s reasonable to register baseline PD to detect any possible change, since the deepening of PD has proved to be a predictive factor of disease development.14,26
Crestal Bone Loss sets another ambiguous point because an adaptive change of the marginal bone level is known to occur afterimplantplacement and restoration.1It’s necessary to agree a baseline for the radiographic evaluation of bone level changes and set an acceptable bone loss rate. Basing on longitudinal clinical studies, it’s rational to chose the time of prosthesis installation as a reference from which the disease can be diagnosed and followed.14 Basing on Albrektson and Zarb review, 1.5mm of bone loss in the first year and less than 0.2mm annually are considered success criteria.1 A CBL exceeding this rate testifies the risk of implant failure. Don't forget that intra-oral x-rays allow to evaluate the interproximal bone level only, missing an appropriate vision of the buccal/lingual sides, where probing becomes essential. Bleeding on Probing is the key parameter for peri-implant disease diagnosis.13 Presence of BOP can be found in 91% of implants with peri-implantitis and its absence is regarded as a reliable predictive parameter of implant health.12
An appropriate diagnosis can be set only if a proper probing is possible. Malpositioning, implant and abutment design (e.g. platform switching), lack of surface smoothness, design, over-contouring and extension of supra structures may make probing difficult and puts the risk of underestimation.14,26 Underestimation of PD can lead to underestimation of CBL.32 If undiagnosed, peri-implantitis may lead to complete failure of osseo integration and implant loss.12 The epidemiology is not comforting:in a recent systematic review the authors concluded that 43% of the implants included in the meta-analysis were affected by mucositis, whereas the prevalence of peri-implantitis was estimated to be 22%.6
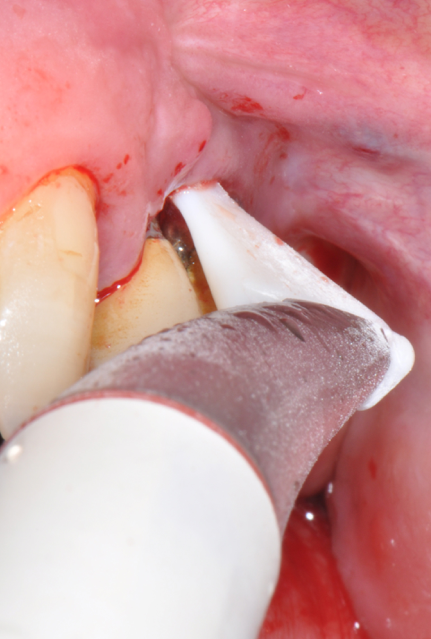
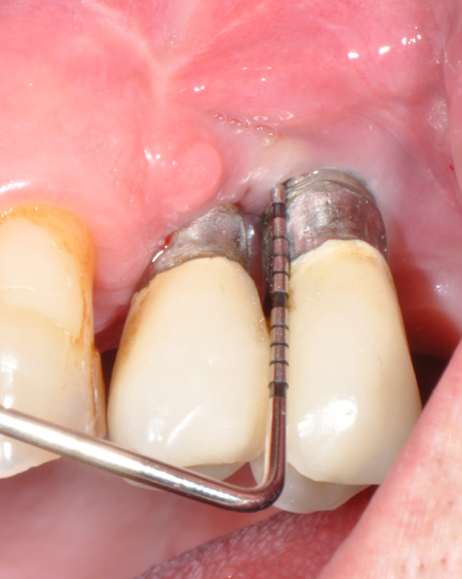
Fig.1: Case 1. Peri-implant probing reveals a PPD of 9mm and pus.
Fig.2: Case 1. BOP starts immediately after probing.
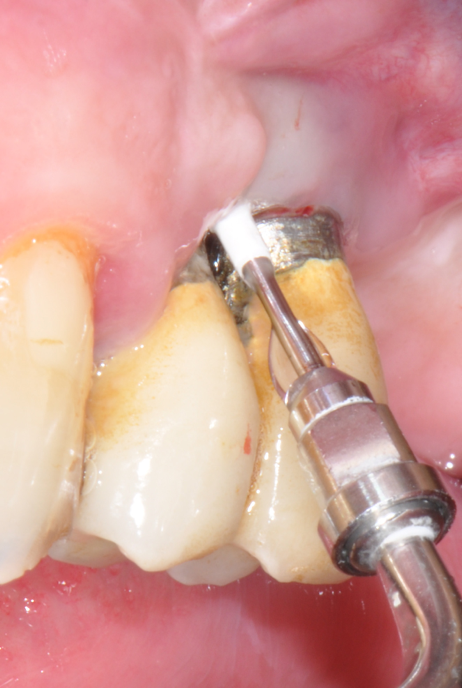
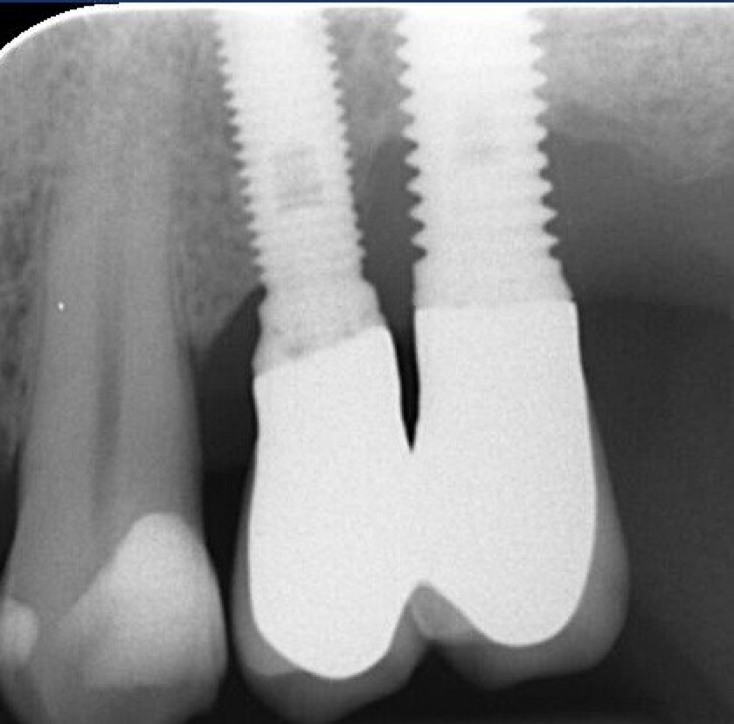
Fig.3: Case 1. X-ray testifying severe peri-implant CBL.
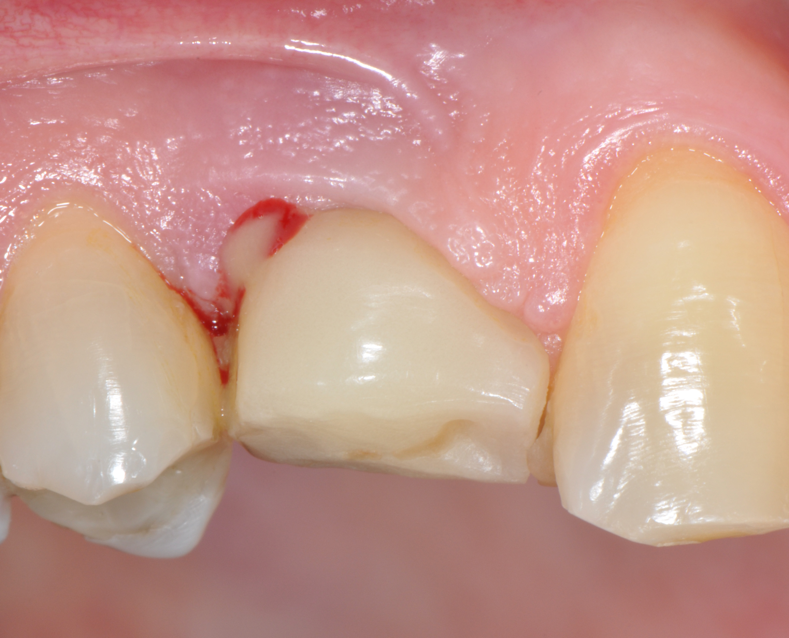
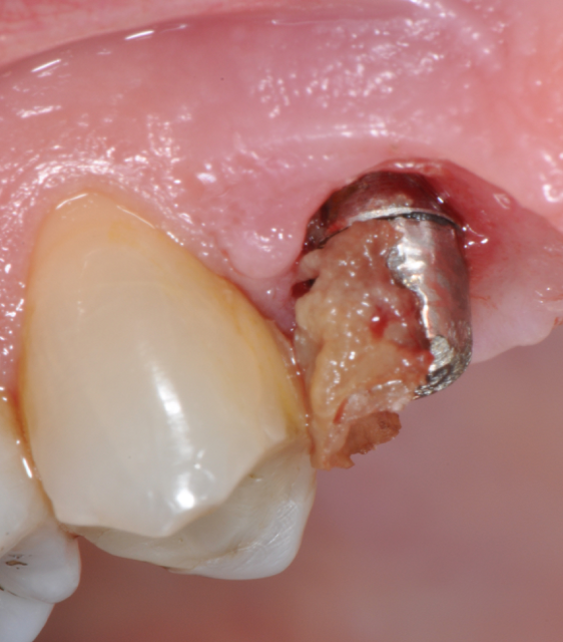
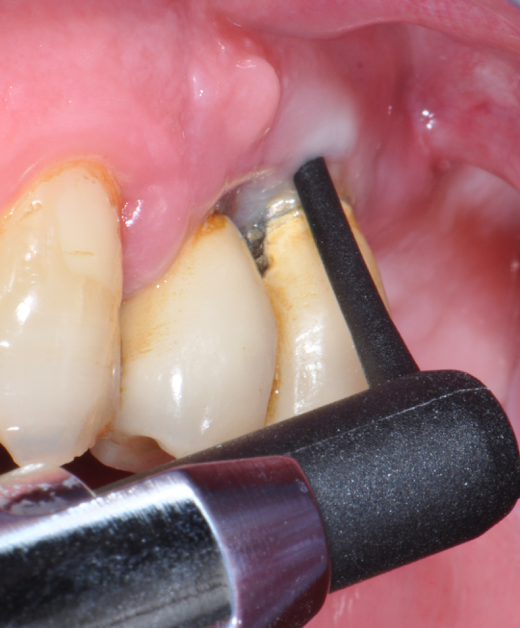
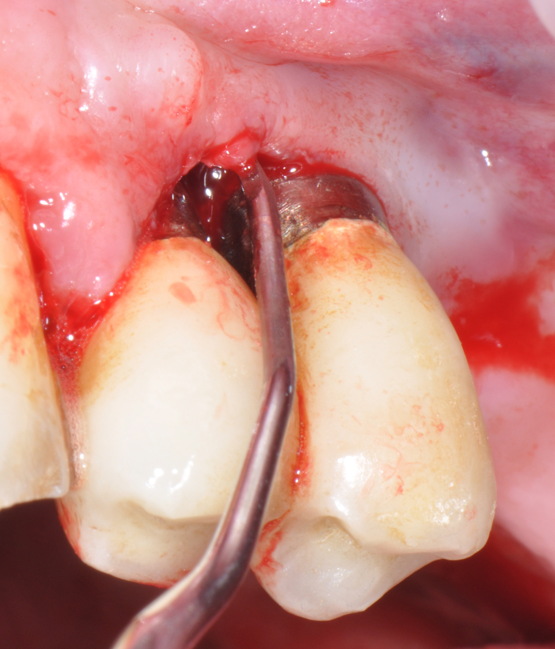
Fig.4: Case 1. Clinical appearance after the prosthetic crown removal.
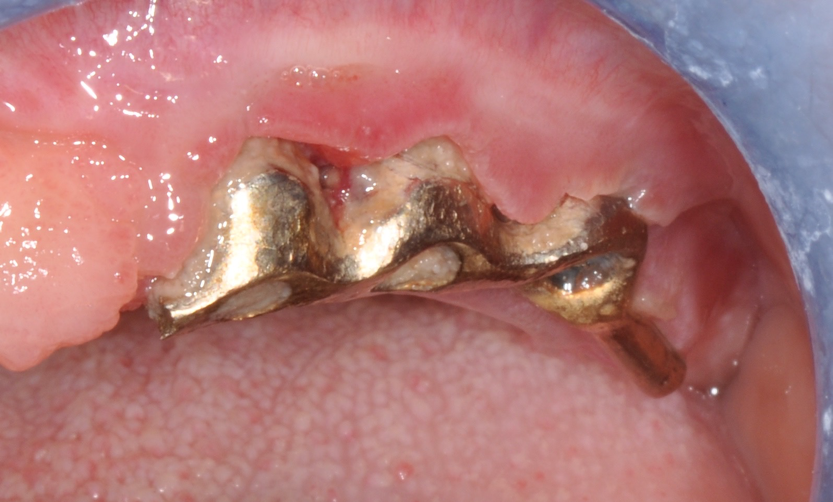
Fig.5: Implant bar with abundant plaque deposits and evident mucositis.
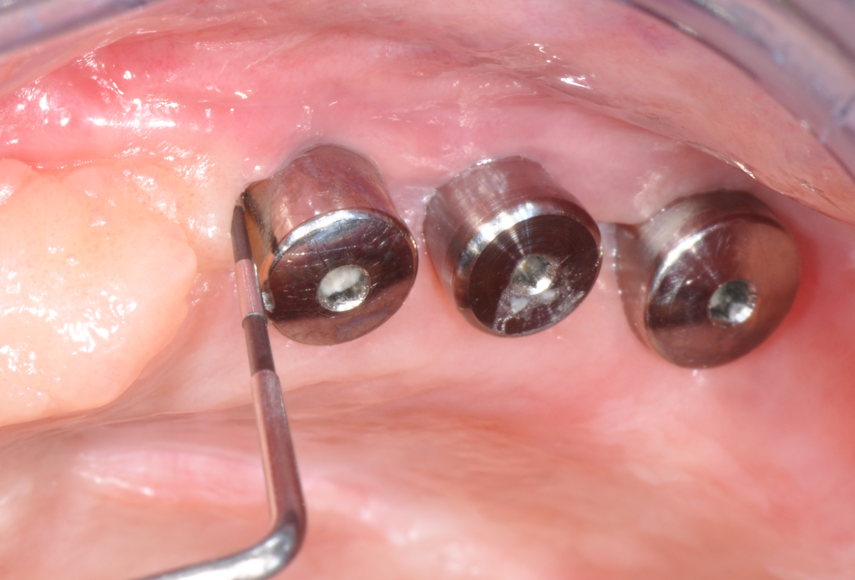
Fig.6: Resolution of mucositis after non-surgical therapy and healing period without bar.
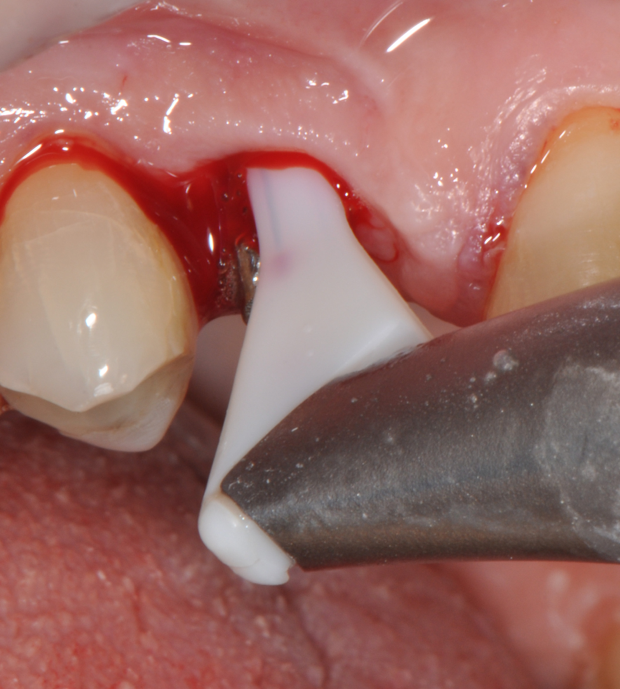
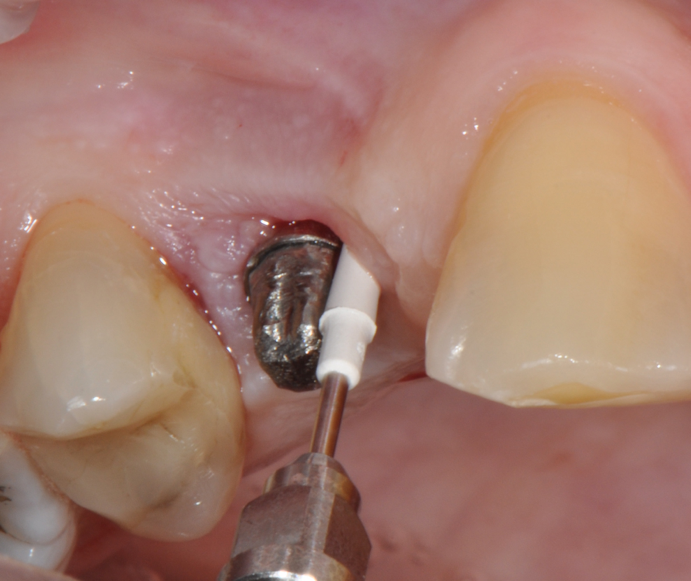
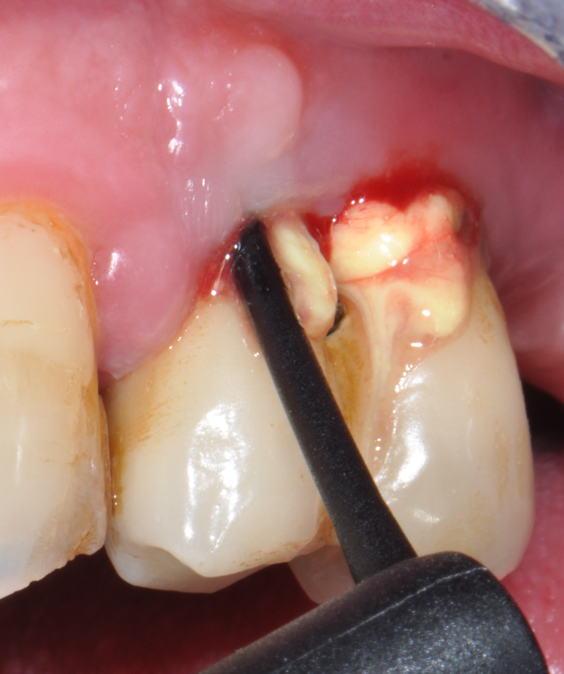
Fig.7: Pocket decontamination with erythritol powder conveyed by sub-gingival tip.
Fig.8: Implant surface debridment with piezo-ceramic device and PEEK tip.
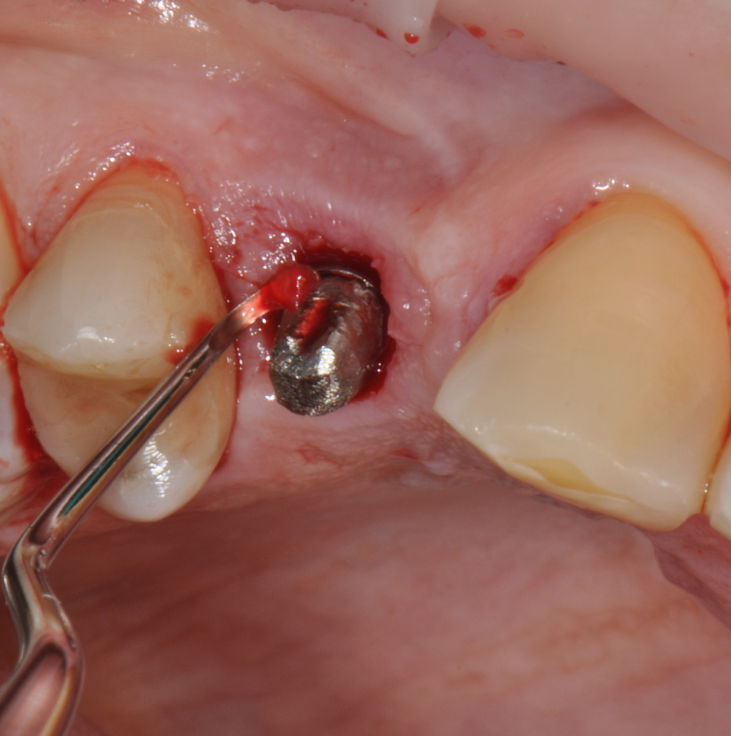
Fig.9: Internal pocket line curettage.
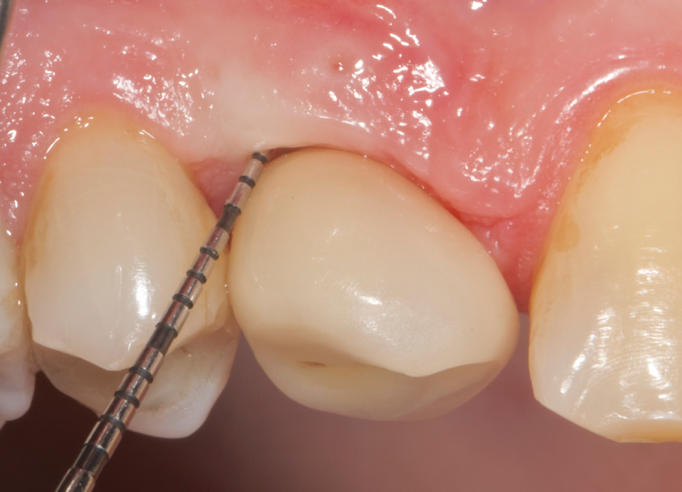
Fig.10: Case 1. Healing at 6 months after MAINST therapy. PPD has decreased to 2mm. BOP and suppuration are absent.
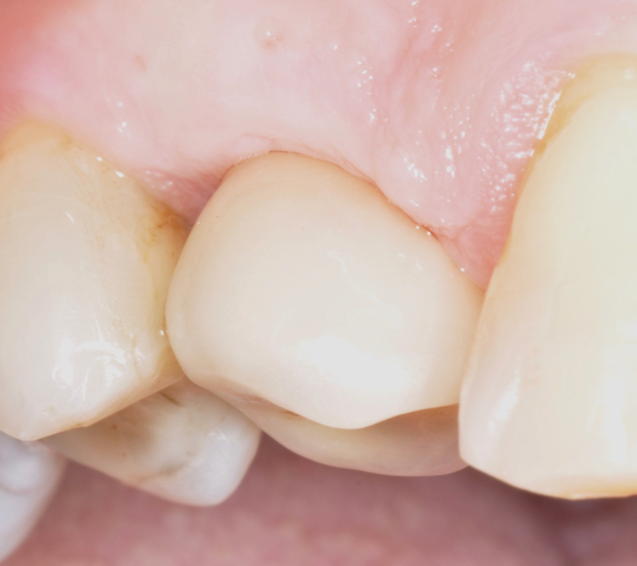
Fig.11: Case 1. Healing at 12 months after MAINST therapy.
Fig.12: Case 2. Baseline. Probing reveals a deep PPD with abundant suppuration and BOP.
Fig.13: Case 2. Baseline. The radiography shows severe peri-implant CBL.
Fig.14: Case 2. First application of doxycycline 14%.
Fig.15: Case 2. Supra-gingival biofilm removal with erythritol powder.
Fig.16: Case 2. Subgingival decontamination with erythritol powder and sub-gingival tip.
Fig.17: Case 2. Implant surface debridment with piezo-ceramic device and PEEK tip.
Fig.18: Case 2. Internal pocket line curettage.
Fig.19: Case 2. Second application of doxycycline 14%.
Fig.20: Case 2. 12 months healing. PPD reduction and BOP absence are noticeable.
Fig.21: Case 2. 12 months radiographic check.
Peri-implantitis lesions are different from periodontal ones, both in their extent and composition of the inflammatory infiltrate.2 Peri-implantitis is known to progress faster than periodontal lesions14 and has a more uncertain response to both surgical and non-surgical treatments.23 This is enough to affirm that prevention is of major importance for the success of implant restorations. The prevention starts with patients framing into risk categories.13 Subjects with a history of periodontitis are at greater risk to develop MBL and peri-implantitis.12 This risk is increased in case of rough implants, poor oral hygiene, smoke habits, diabetes and poor metabolic control.12,14,21 The clinician must be able to diagnose and treat periodontal disease and have the duty to work on patients’ habits, giving them support in a change that can bring benefits not only to the implant therapy but to their health as well.13
Second step of prevention can be carried out during the surgical phase: a correct positioning of the fixture can help the technician in constructing a correct prosthesis and, consequently, the periodontologist in checking the implant health, the hygienist in cleaning effectively the peri-implant area13 and the patient in keeping an high standard home-care. An ineffective care leads to the development of inflammatory reactions that can be kept hidden under the the prostheses and be unrevealed until their removal. (Fig.4) Particular attention should be given to reach an appropriate amount of keratinized peri-implant tissue: its presence can be beneficial for the maintenance of an adeguate oral hygiene.13 Long abutments and implant placement at sub-mucosal level cannot be considered a good choice from the periodontal point of view since they may create a deep probing depth since the very beginning of the implant-born restorations’ life.13
Third milestone of the peri-implantitis prevention is Supportive Periodontal Therapy (SPT): the lack of a regular and effective SPT is a risk factor for the development of peri-implantitis.21 Every recall should be accompanied by a proper examination and probing13 to detect and effectively treat any case of peri-implant mucositis, since it can early progress to peri-implantitis.14 Sometimes it might be necessary to remove the overlying prostheses in order to achieve a more effective treatment and, in some cases, a better resolution of the inflammatory disease. (Fig.5,6)
The objective of the SPT should be the absence of peri-implant inflammation witnessed by absence of BOP.27
But what should we do in case peri-implantitis diagnosis?
Being an infective pathology, biofilm and calculus removal is the key of peri-implant treatment.13 A gold standard non-surgical treatment still does not exists.27 Up to now no clinically relevant advantage of one treatment over the other can be found8 and only limited improvements accompanied by a tendency for recurrence have been reported. 9 What has been happening during the last decades is the transposition of periodontal therapy strategies and technologies to the implant world. The use of curettes and mechanical devices can be reasonable since it’s proved that peri-implant diseases are caused by a complex biofilm that has to be disrupted14 but becomes disputable given the structural differences between a tooth and a implant. Scaling and Root Planing makes little sense on a titanium surface with its particular micro and macro structure. An implant should not be planed but detoxified and decontaminated without alteration of its smooth and rough surfaces and with recover of the biocompatibility.16 Erosion with liberation of ions and metal particles is an underestimated issue in dentistry. Wear debris have been described to be oneof the responsible factors for aseptic loosening of orthopaedic implants.33 They can be phagocytized by macrophages, inducing the expression of pro-inflammatory cytokines activating osteoclasts maturation.19On the surface of titanium implants we can find a self-repairable layer of TiO2 that shows an high chemical stability and prevent the diffusion of metallic ions. Scratching of the implant or abutment surface could lead to the temporary removal of the TiO2 layer and to release of metal particles.3 Fretwurst et al.11 analysed bioptic samples of bone and soft tissues from patients with severe peri-implantitis. In 75% of the biopsies it was possible to detect titanium accompanied by pro-inflammatory macrophages. Furthermore, the alteration of the oxide layer and the contamination of the surface by instrument’s debris result in a impaired cell adhesion and implant biocompatibility.10,17 In some in vitro studies, implant surfaces treated with stainless-steel curettes show a significantly lower number of attached fibroblast compared to untreated controls.17 Ultrasonic scalers with metal tips are effective in removing plaque from implant surfaces16but cause damages mostly to the smooth surfaces, increasing the roughness and the possibility of new biofilm formation.15
This is the reason why different materials curettes made have been introduced to not damage the implant surface (titanium-coated, carbon fibre, teflon, plastic). Same happened with ultrasonic devices: etherketone-coated tips have been proposed as an efficient scaling mean. Fox et al.10 showed that plastic and titanium-allow curette produce significantly lower roughness on titanium surface compared to steel ones. Unfortunately, the softer the material, the more limited is the debridment power. Different non-metal curettes were found to be ineffective in removing bacteria and calcified deposits from smooth as well as rough titanium surfaces.16 They also show lack of flexibility which prevents an adeguate cleaning of the threads. Ultrasonic scalers with non-metal tips seem to be effective in removing bacteria from smooth surfaces but show controversial results with rough surfaces.16
In order to overcome these limitations, coadjuvants and new technologies have been introduced and combined. Air-polishing devices aim to an easier and more efficient biofilm removal. Abrasive powders are expected to be more efficient in reaching the inner part of the threads and the smallest anfractuosity other than being respectful of the metal surfaces.15 Sodium bicarbonate is proved to be highly effective in removing bacteria, in particular from rough implant surfaces16and to be more effective than plastic manual and mechanical instruments, independent of the surface characteristics.16 The downside is that sodium bicarbonate can be harmful for soft tissues and can increase roughness of smooth surfaces.16 This problem has been overcome thanks to low-abrasiveness powders such as glycine and erythritol, proven to be respectful of oral soft tissues.5 Good in-vitro results are reported: glycine seems to be effective in removing bacteria from both smooth and rough surfaces.16 Repeated use of glycine powder was not associated with any surface alterations,16 making its use feasible for life-long implant maintenance. Schmage et al.28 proved glycine powder to be as effective as ultrasonic instruments with PEEK tip in cleaning both smooth and structured surfaces. Drago et al.7 analysed the in-vitro effect of erythritol powder finding that it shows even a stronger antimicrobial and antibacterial activity than glycine. The detoxifying. Erythritol powder has a lower granulometry although the abrasive power is high. This may help reaching the micro-anfractuosity of the implant and, in conjunction with the antimicrobial activity, help detoxifying the surface.
Schmidt et al.29 analysed the effects of different instrumentations (stainless steel and plastic curettes, stainless steel and plastic-coated ultrasonic devices, two types of glycine powders and one of erythritol) on implant necks through a scanning electron microscope. They found out that air-polishing treatment resulted in the least surface modifications. Amongst the powders tested, the erythritol was proven to be the most respectful of the implant surface.
Furthermore, the introduction of specifically designed flexible nozzles able to reach the deeper portion of the pockets has increased the decontamination power of this kind of devices. Ronay et al.24 in a in-vitro study simulated different peri-implant defect morphologies around rough implants with simulated biofilm and tested the cleaning effectiveness of steel curette, ultrasonic device with steel tip, air-powder abrasive device with glycine powder and nozzle for sub-gingival use. The air-abrasive device provided a superior cleaning efficacy, followed by ultrasonic instrumentations.
The major advantage of the sub-gingival nozzles is the flexibility that eases the access to the peri-implant pockets and to the implant surfaces, mostly when the access is hindered and the removal of the prostheses is not possible.
Even if the in-vitro results are encouraging, in-vivo evidence is still insufficient. Sahm et al.25 in a randomized controlled clinical trial showed that the treatment of initial/moderate peri-implantitis through an air-abrasive device with glycine powder can achieve the same PD reduction of carbon curettes and chlorexidine digluconate. It could also achieve a significantly higher BOP reduction. Randomized controlled clinical trials are required to asses the real in-vivo efficacy of air-polishing devices for the resolution of peri-implantitis, focusing on severe cases.
Antibacterial and antiseptic molecules have been proposed to boost the bacterial elimination and to help decontaminating the implant porous surface. Chlorexidine has shown to be ineffective in peri-implant lesions decontamination. Porras et al.20 could not find any PD reduction and only a limited BOP reduction after additional use of local 0.12% chlorexidine irrigation and gel plus 10 days of 0.12% chlorexidine mouth-rinse. Antibiotics constitute an additional option. Since peri-implantitis isa very localized disease, we wouldn’t take into consideration systemic antibiotic therapy with all the side effects it can bring. It’s important to notice that, to date, there are no controlled clinical trials evaluating the effects of any systemic antibiotic therapy.9 Locally delivered antibiotics can be released in a high dose of for many days, killing the bacteria in the un-removed biofilm. Tetracyclines have been widely investigated in periodontology given their broad action spectre. Mombelli et al.18 tested locally delivered 25% tetracycline as monolithic ethylene vinyl acetate fibers to be located around implants after a scaling phase with plastic curette and to be removed 10 days after. Clinical, radiographic and microbiological parameters improved in a good part of the subjects. Unfortunately, the lack of control group does not allow to understand the real magnitude of the antibiotic action. Amongst the difficulties met by the authors, it’s notable the struggle in assuring a contact between the fibers and all the implant surface, in particular in narrow and deep defects. The use of different biodegradable carriers can give a better and easier contact with the implant structure and can cut out the need of fibers removal. Renvert et al.22 tested a single dose of locally delivered minocycline as a coadjuvant of manual debridment with curettes, compared to chlorexidine gel application. The additional effect of minocycline was small but significantly higher both on PD and BOP. Butcher et al.4 investigated biodegradable slow-release 8.5% doxycycline as an adjunction to debridment with plastic curettes plus motivation and oral hygiene instructions. The results were promising showing a significantly greater gain in mean attachment level, PD and BOP improvement for the doxycycline group. In conclusion, doxycycline seems to be the most effective local antibiotic available.
Schwarz et al.30,31 summarised the most recent evidence about peri-implant disease treatment through plaque removal and adjunctive or alternative measures. Regarding peri-implantitis, a meta-analysis showed that glycine powder as an alternative biofilm removal measureand local antibiotic therapy as an adjunct to mechanical debridment allow to achieve a higher BOP reduction over respective control treatments. These are the reasons why we decided to bring in our clinical experience the use of PEEK ultrasonic tips associated with supra- and sub-gingival air-polishing systems with glycine or erythritol powder and a controlled-release 14% doxycycline hyclate (Ligosan®).
So far, there is no scientific evidence supporting the efficacy of this coadjuvant. The tested protocol consist of a Multiple Anti Infective Non Surgical Therapy (MAINST) that involves the use topical 14% doxycycline to solve the peri-implantitis acute phase and, after 7 days, a session of Full Mouth Air Polishing Therapy (FM-EPAPT) through erythritol powder (Fig.7), a piezo-ceramic device with a PEEK tip (Fig.8), the curettage of internal pocket line (Fig.9) and a second application od Doxy. The patients were further followed with quarterly maintenance sessions carried on with the same FM-EPAPT protocol. Up to 12 months BOP and mean PD decreased significantly and successfully, accompanied by a gain of attachment level up to 12 months. The first case-series about MAINST is waiting to be published and the results are encouraging. Figure 10 and 11 show the healing at 6 and 12 months after MAINST protocol of the peri-implantitis case displayed at the beginning of this article (Fig.1,2,3,4) and figure 12-21 show a complete MAINST case.
- A first effective implant pocket decontamination with respect of soft tissues though a topical antibiotic to solve the acute phase of peri-implantitis;
- A decontamination and detoxification phase though erythritol powder and piezo-electric device that covers the entire oral cavity (FM-EPAPT);
- A strict professionalmaintenance protocol based on EPAPT.
- A stricthome caremaintenance protocol.
The home-care maintenance is fundamental for the maintenance of the treatment results.9 The facilities given to the patients include: sonic tooth brush, interdental brushes, floss and air-floss (Philips Sonicare AirFloss Ultra).
Bibliography
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants, 1986;1:11–25.
- Berglundh, T., Zitzmann, N. U. and Donati, M. Are peri-implantitis lesions different from periodontitis lesions?. Journal of Clinical Periodontology, 2011;38:188–202. doi:10.1111/j.1600-051X.2010.01672.x
- Bhola R, Bhola SM, Mishra B, Olson DL. Corrosion in titanium dental implants/prostheses - a review. Trends Biomater Artif Organs, 2011;25(1), 34–46
- Buchter A, Meyer U, Kruse-Losler B, Joos U, Kleinheinz J. Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg, 2004;42: 439–444
- Bühler J, Amato M, Weiger R, Walter C. A systematic review on the effects of air polishing devices on oral tissues.Int J Dent Hygiene, 2015 DOI:10.1111/idh.12120
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. Journal of Clinical Periodontology,2015;42:S158—S71.
- Drago L , Del Fabbro M, Bortolin M, Vassena C, De Vecchi E, Taschieri S. Biofilm removal and antimicrobial activity of two different air-polishing powders: an in vitro study. J Periodontol, 2014; Nov;85(11):e363–9. doi: 10.1902/jop.2014.140134. Epub 2014 Jul 25.
- Esposito M, Grusovin MG, Tzanetea E, Piattelli A, Worthington HV. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database of Systematic Reviews, 2010;Issue 6. Art. No.: CD004970. DOI: 10.1002/14651858.CD004970.pub4.
- Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000, 2014;66: 255– doi:10.1111/prd.12049
- Fox SC, Moriarty JD, Kusy RP. The Effects of Scaling a Titanium Implant Surface With Metal and Plastic Instruments: An in Vitro Study. J Perio, 1990;61(8):485–490 doi: 10.1902/jop.1990.61.8.485
- Fretwurst T, Buzanich G, Nahles S, Woelber JP, Riesemeier H, Nelson K. Metal elements in tissue with dental peri-implantitis: a pilot study. Clin Oral Impl Res; 2016;27:1178–1186 doi: 10.1111/clr.12718
- Heitz-Mayfield LJA. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol, 2008;35(Suppl. 8):292–304. doi: 10.1111/j.1600-051X.2008.01275.x
- Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, Figuero E, Giovannoli JL, Goldstein M, Lambert F, Ortiz-Vigon A, Polyzois I, Salvi GE, Schwarz F, Serino G, Tomasi C, Zitzmann NU. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol, 2015;42(Suppl. 16):S152–S157. doi: 10.1111/jcpe.12369
- Lang NP, Berglundh T on Behalf of Working Group 4 of the Seventh European Workshop on Periodontology: Periimplant diseases: where are we now? – Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol, 2011;38(Suppl.11):178–181. doi: 10.1111/j.1600-051X.2010.01674.x.
- Louropoulou A, Slot DE,van der Weijden Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Impl Res, 2012;23: 643–658.
- Louropoulou A, Slot DE, Van der Weijden F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Impl Res, 2014:25:1149–1160 doi: 10.1111/clr.12224
- Louropoulou A, Slot DE, Van der Weijden F. Influence of mechanical instruments on the biocompatibility of titanium dental implants surfaces: a systematic review.
Clin Oral Impl Res, 2015;:841–850. doi: 10.1111/clr.12365
- Mombelli A, Feloutzis A, Brägger U, Lang NP. Treatment of peri-implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clin Oral Impl Res, 2001;12: 287–294
- Obando-Pereda GA, Fischer L,Stach-Machado Titanium and zirconia particle- induced pro-inflammatory gene expression in cultured macrophages and osteolysis, inflammatory hyperalgesia and edema in vivo. Life Science, 2014;97: 96–106.
- Porras R, Anderson GB, Caffesse R, Narendran S, Trejo Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Perio, 2002;73, 1118–1125.
- Quirynen M, Abarca M, Van Assche N, Nevins M,van Steenberghe D. Impact of supportive periodontal therapy and implant surface roughness on implant outcome in patients with a history of periodontitis. Journal ClinPeriodontol, 2007;34, 805–815.
- Renvert S, Lessem J, Dahlén G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: a randomized clinical trial. J Clin Periodontol 2006; 33: 362–369. doi: 10.1111/j.1600-051X.2006.00919.x.
- Renvert S, Polyzois IN. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Perio 2000, 2015;68(1):369–404.
- Ronay V, Merlini A, Attin T, Schmidlin PR, Sahrmann P. In vitro cleaning potential of three implant debridement methods. Simulation of the non-surgical approach. Clin Oral Impl Res, 2016;00:1–6 doi: 10.1111/clr.12773
- Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol, 2011;38: 872–878
- Salvi GE, Lang NP. Diagnostic parameters for monitoring peri-implant conditions. Int J Oral Maxillofac Implants, 2004;19(suppl):116–127
- Sanz M, Chapple IL, on behalf of Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol, 2012;39(Suppl. 12):202–206. doi: 10.1111/j.1600-051X.2011.01837.x
- Schmage P, Thielemann J, Nergiz I, Scorziello TM,Pfeiffer P. Effects of 10 cleaning instruments on four different implant surfaces. The International Journal of Oral & Maxillofacial Implants, 2012;27: 308–317
- Schmidt KE, Auschill TM, Heumann C, Frankenberger R, Eick S, Sculean A, Arweiler NB. Influence of different instrumentation modalities on the surface characteristics and biofilm formation on dental implant neck, in vitro. Clin Oral Impl Res 2016;00:1–8
- Schwarz F, Schmucker A, Becker J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis.Int J Implant Dent.2015 Dec;1(1):22. Epub 2015 Aug 13.
- Schwarz F, Becker K, Renvert S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: a systematic review. J Clin Periodontol 2015; 42: 951– doi:10.1111/jcpe.12454.
- Serino G, Turri A, Lang NP. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin Oral Impl Res, 2013;24:91–95 doi: 10.1111/j.1600-0501.2012.02470.x
- Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C.Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthopaedica, 2006;77: 177–197.
Tabitha Acret explains how Guided Biofilm Therapy has revolutionised how she treats orthodontic patients
The Straumann Group held Esthetic World 2022 in Dubai in January. In this interview, Dental Tribune MEA speaks to Wolfgang Becker, Executive Vice President,...
Specialist Periodontist, Dr Jeremy Vo, explains how he has embraced AIR-FLOW® technology in the management of Peri-Implantitis
The Ormco Forum Dubai 2018 took place from 06 to 08 December 2018 at Palazzo Versace, Dubai, UAE
Dental professionals across the Middle East have undoubtedly heard of Beverly Hills Formula, a brand that has successfully carved their name as unrivalled ...
Capabilities in dentistry have evolved at an astonishing rate over the past several years, and the transformations are by no means slowing down.
Oral Care experts Beverly Hills Formula have proven once more that their safe, stylish and affordable range of teeth whitening products continue to be firm ...
Today’s technological and design innovations have seen the humble dental chair evolve into state-of-the-art treatment centers, putting ergonomics and ...
By supplying innovative equipment, Dentsply Sirona International Special Clinic Solutions (ISCS) empowers dental professional to provide better dental care...
Accelerated precision for perfect polymer restorations
Live webinar
Tue. 24 February 2026
10:00 pm UAE (Dubai)
Prof. Dr. Markus B. Hürzeler
Live webinar
Wed. 25 February 2026
12:00 am UAE (Dubai)
Prof. Dr. Marcel A. Wainwright DDS, PhD
Live webinar
Wed. 25 February 2026
8:00 pm UAE (Dubai)
Prof. Dr. Daniel Edelhoff
Live webinar
Wed. 25 February 2026
10:00 pm UAE (Dubai)
Live webinar
Thu. 26 February 2026
5:00 am UAE (Dubai)
Live webinar
Tue. 3 March 2026
8:00 pm UAE (Dubai)
Dr. Omar Lugo Cirujano Maxilofacial
Live webinar
Wed. 4 March 2026
5:00 am UAE (Dubai)
Dr. Vasiliki Maseli DDS, MS, EdM



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 International / International
International / International
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس